Discriminating between viable (live) and non-viable (dead) cells is important to flow cytometric analysis. Dead cells in your samples can non-specifically bind to your antibodies, resulting in false positives and, ultimately, inaccurate results.
Discrimination between live and dead cells in flow cytometric analysis can be carried out with the use of the 7-AAD Viability Dye or Propidium Iodide (PI) Staining Solution. The 7-AAD or PI will mark the non-viable cells by binding to the nuclei of the dead cells. The nucleic acid of the viable cells in your sample will not be accessible to the dye and will not be stained. When analyzing the flow cytometry data collected, gate out all cells stained with the viability dye.
When staining intracellular proteins, discriminate your live and dead cell populations using Fixable Viability Dye eFluor 450, Fixable Viability Dye eFluor 660, or Fixable Viability Dye eFluor 780. These eFluor Fixable Viability Dyes are permanent dyes, and will not wash out of cells as they are prepared for analysis. For more information on the eFluor Fixable Viability Dyes for flow cytometry, please see our Functional Dyes & Reagents webpage.
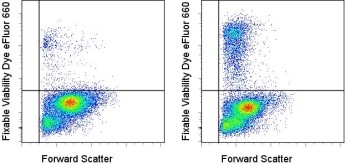
Fixable Viability Dye eFluor 660 Data

BALB/c thymocytes were uncultured (left) or cultured overnight at 37°C (right) and then stained with 1 uL of Fixable Viability Dye eFluor 660 per mL of thymocytes resuspended at 5x10e6 ells/mL in PBS. Total cells were used for analysis.




Leave a reply