Cancer is one of the major health problems. Although traditional therapies have been effective in cancer treatment, they often have adverse side effects due to their nonspecific action on both normal and tumor cells. Therefore, targeted treatment strategies using small molecule inhibitors are being extensively studied. These compounds are usually ≤500Da size and are often administered orally. In principle, small molecule compounds can be developed to target any portion of a molecule, regardless of the target’s cellular location. In this blog entry, we provide a summary of the small molecule inhibitors (SMIs) used in the cancer therapy.
- 1) Introduction
- 2) Small Molecule Inhibitors
- 3) List of small molecule inhibitors for cancer therapy
- 4) Limitations of the Small Molecules
- 5) Conclusions
- 6) References
1) Introduction
The traditional means of cancer management are chemotherapy, radiation therapy and surgery. Chemotherapy that involves the use of a single drug at a time (single-agent chemotherapy) or several drugs (combination therapy) usually target killing of rapidly dividing cancer cells. To overcome the drawbacks of traditional cancer therapies, the approach has been to search for specific molecular targets for selective elimination of cancer cells. Such targeted therapy would conceptually be more specific than the traditional non-targeted therapy. The two main approaches of specific molecular targeting available for use in clinical practice are small molecule agents and monoclonal antibodies (mAbs) [1]. When compared to mAbs which are usually large molecular weight proteins of around 150kDa, small molecule cancer drugs are much smaller in size (≤500Da) and thus can translocate through plasma membranes. On the contrary, the mAbs can only act on molecules that are expressed on the cell surface [8]. The small molecule inhibitors are also comparatively cost effective and are amenable to oral administration while the mAbs are mostly administered intravenously [2].
2) Small Molecule Inhibitors
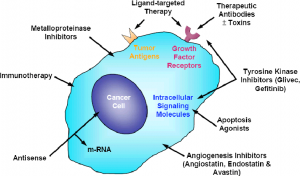
Small molecule cancer drugs, because of their small size, have been successfully used to target the extracellular, cell surface ligand-binding receptors as well as the intracellular proteins, including anti-apoptotic proteins that play a key role in transducing downstream signalling for cell growth and metastasis promotion. Most of these drugs inhibit critical cancer targets such as serine/threonine/tyrosine kinases, matrix metalloproteinases (MMPs), heat shock proteins (HSPs), proteosome and other proteins playing a role in signal transduction pathways.
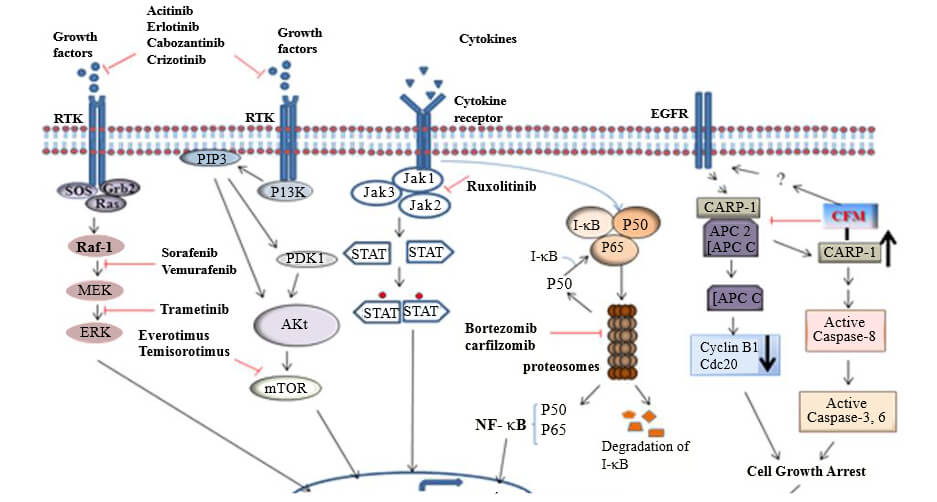
3) List of Small Molecule Inhibitors for cancer therapy
Many cancer therapeutic drugs have been approved for use and several more are being studied in clinical trials.
| Molecular Target | Small Molecule | Specific Target | Cancer targeted | Clinical Status |
| Tyrosine & Serine/Threonine kinases | Imanitib | Bcr-Abl | Philadelphia chromosome-positive chronic myelogenous leukemia | FDA approved in 2001 |
| Gefitinib | EGFR | Non-small Cell Lung Cancer | FDA approved in 2003 | |
| Erlotinib | EGFR | Non-small Cell Lung Cancer, Pancreatic cancer | FDA approved in 2005 | |
| Sunitinib | VEGFR2, RET, PDGFR, FLT-3, KIT, CSF-1 | Renal cell carcinoma | FDA approved in 2006 | |
| Lapatinib | EGFR, HER2/neu | Breast cancer | FDA approved in 2007 | |
| Nilotinib | Bcr-Abl, KIT, LCK | Chronic myelogenous leukemia | FDA approved in 2007 | |
| Sorafenib | B-Raf, VEGFR2, EGFR, PDGFR | Hepatocellular carcinoma | FDA approved in 2007 | |
| Temsirolimus | mTOR | Renal cell carcinoma | FDA approved in 2007 | |
| Everolimus | mTOR | Renal cell carcinoma | FDA approved in 2009 | |
| Pazopanib | c-KIT, FGFR, PDGFR, VEGFR | Renal cell carcinoma, soft tissue sarcoma | FDA approved in 2009 | |
| Crizotinib | HGFR | Non-small cell Lung Cancer | FDA approved in 2011 | |
| Ruxolitinib | Jak1, Jak2 | Primary Myelofibrosis | FDA approved in 2011 | |
| Axitinib | VEGFR1-3, cKIT, PDGFR | Renal cell carcinoma | FDA approved in 2012 | |
| Bosutinib | Src, Bcr-Abl | Philadelphia chromosome-positive chronic myelogenous leukemia | FDA approved in 2012 | |
| Cabozantinib | c.Met, VEGFR2 | Medullary thyroid cancer | FDA approved in 2012 | |
| Ponatinib | Bcr-Abl | Chronic myeloid leukemia, acute lymphoblastic leukemia | FDA approved in 2012 | |
| Regorafenib | VEGFR1-3, c-Kit, TIE-2, PDGFR-β, FGFR-1, RET, Raf-1, BRAF | Metastatic colorectal cancer | FDA approved in 2012 | |
| Ibrutinib | Bruton’s tyrosine Kinase | Mantle cell lymphoma | FDA approved in 2013 | |
| Trametinib | MEK | B-RAF 600E/K-mutant metastatic melanoma | FDA approved in 2013 | |
| Perifosine | Akt | Colorectal Cancer, Multiple Myeloma | Approved as Orphan drug for multiple myeloma and neuroblastoma. Phase II of clinical trial for metastatic colon cancer. |
|
| Proteosomes | Bortezomib | 26S proteosome | Multiple myeloma | FDA approved in 2003 |
| Carfilzomib | 20S proteosome | Multiple myeloma | FDA approved in 2012 | |
| MMPs & HSPs | Batimastat | Broad spectrum MMPs | Various tumors | Yet to be approved |
| Ganetespib | HSP 90 | Multiple cancers | Phase III of clinical trial | |
| NVP-AUY922 | HSP 90 | Various tumors | Phase II of clinical trial | |
| Apoptosis | Obatoclax | Bcl-2 family of proteins | Leukemia, lymphoma, myelofibrosis | Phase II of clinical trial |
| Navitoclax | Bcl-xL, Bcl-2 and Bcl-w | Lymphoma and Chronic Lymphocytic Leukemia | Phase II of clinical trial |
4) Limitation of the use of Small Molecules
More than 20 small molecule drugs have been approved for clinical use and are being successfully used in cancer treatment. Still, there are certain limitations that are to be considered and overcome while designing more active drugs so as to reduce the failure rates of the drugs at the clinical level. Certain small molecule inhibitors bind to multiple molecular targets including cell surface receptors and other intracellular proteins thus increasing the risk of toxicity [3]. Most of the small molecule drugs have a short life span, thus requiring daily dosing which is not the case with therapeutic mAbs that have a longer life span [3].
5) Conclusions
Over the last few decades, the success of small molecule cancer drugs over conventional chemotherapy has been clearly demonstrated. Majority of the inhibitors that have been developed and currently in clinical use target the kinases, which include the receptor molecules as well as downstream regulators [4]. Labclinics includes in its catalog, the catalog from ApexBio, a leading provider of peptides, small molecules and laboratory reagents for biomedical research. Its main product line is apoptosis and epigenetics. ApexBio also provides peptide synthesis.
Soy un bloque de texto. Haz clic en el botón Editar para cambiar este texto. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
6) References
[1] Wu HC, Chang DK, Huang CT (2006) Targeted Therapy for Cancer. Journal of Cancer Molecules 2: 57-66
[2] Imai K, Takaoka A (2006) Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 6: 714-727.
[3] Curigliano G, Criscitiello C. (2014) Successes and limitations of targeted cancer therapy in breast cancer. Prog Tumor Res 41: 15-35.
[4] Bari SB, Adhikari S, Surana SJ (2012) Tyrosine Kinase Receptor Inhibitors: A New Target for Anticancer Drug Development. Journal of Pharma Sci Tech 1: 36-45.





Leave a reply