All immunostaining and immunoassay applications depend on the use of sensitive and highly specific antibodies, yet despite cross-reactivity, batch-to-batch variability, and the use of antibodies in untested applications have all been blamed for causing potentially devastating effects on research.
- 1) Check that the antibody is suitable for the chosen application
- 2) Select an appropriate host species and clonality
- 3) Choose a suitable secondary antibody
- 4) Refer to the literature
- 5) Study the product datasheet
- 6) Examine protocols for optimal results
- 7) Handle the antibody correctly
- 8) Always include relevant experimental controls
1) Check that the antibody is suitable for the chosen application
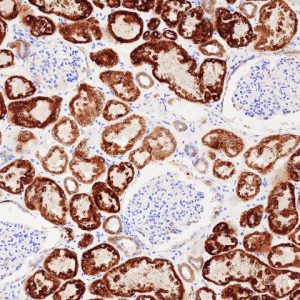 It should never be assumed that an antibody that works for one application will work in every possible scenario. For example, an antibody may recognize an epitope within a frozen tissue section in an IHC study, yes this epitope may not be accessible in a sample that has been reduced and denatured for Western blot analysis. The product datasheet supplied with the antibody should detail all applications in which it has been tested. Those applications in which it has produced the expected pattern of staining should be included, along with batch-specific data.
It should never be assumed that an antibody that works for one application will work in every possible scenario. For example, an antibody may recognize an epitope within a frozen tissue section in an IHC study, yes this epitope may not be accessible in a sample that has been reduced and denatured for Western blot analysis. The product datasheet supplied with the antibody should detail all applications in which it has been tested. Those applications in which it has produced the expected pattern of staining should be included, along with batch-specific data.
2) Select an appropriate host species and clonality
It’s important that the host species of the primary antibody is different than the species of the test sample to avoid cross-reactivity of secondary antibodies with endogenous immunoglobulins in the sample material. It’s also prudent to consider the clonality of the primary antibody. Monoclonal antibodies are homogeneous, recognizing a single antigenic epitope and offering the advantages of low background staining, consistent experimental results, and reagent availability for the duration of a research project. Disadvantages may include unsuitability for antigen detection across multiple species, and vulnerability to epitope loss due to sample treatment methods. Polyclonal antibodies bind multiple epitopes, providing signal amplification and a greater tolerance of antigen changes, yet they may also be associated with unwanted background staining and batch-to-batch variability.
3) Choose a suitable secondary antibody
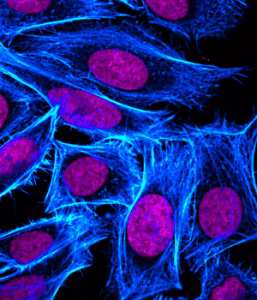 Secondary antibodies, raised against the host species of the primary antibody, are available in many formats and should be selected according to the application. They afford a simple method of signal amplification via the binding of multiple secondaries to a single primary antibody, yet their use may also impose limitations on an experiment. For example, multiplexing experiments that rely on secondary antibodies for detection require that each primary antibody is raised in a different host, and in some instances directly conjugated primary antibodies may be preferred. Primary antibody conjugates can increase experimental -plex as well as reduce the number of steps within an immunostaining protocol yet, as with all antibodies, rigorous validation is essential to ensure that cross-reactivity does not generate misleading results.
Secondary antibodies, raised against the host species of the primary antibody, are available in many formats and should be selected according to the application. They afford a simple method of signal amplification via the binding of multiple secondaries to a single primary antibody, yet their use may also impose limitations on an experiment. For example, multiplexing experiments that rely on secondary antibodies for detection require that each primary antibody is raised in a different host, and in some instances directly conjugated primary antibodies may be preferred. Primary antibody conjugates can increase experimental -plex as well as reduce the number of steps within an immunostaining protocol yet, as with all antibodies, rigorous validation is essential to ensure that cross-reactivity does not generate misleading results.
4) Refer to the literature
PubMed and CiteAb are excellent resources for finding out which antibodies other researchers are using. They can also be mined to determine which applications and experimental conditions have been successful with a particular antibody product.
5) Study the product datasheet
As well as providing information regarding the applications in which an antibody has been validated, product webpages and product datasheets contain a wealth of additional detail. This typically includes a description of the immunogen, the nature of the epitope if this has been mapped, the host species, the antibody isotype, cross-species reactivity (either tested or predicted based on sequence homology), and recommended starting dilutions. If a manufacturer has not evaluated an antibody in a certain application, this does not mean that it will not work. Conditions such as antibody dilution, incubation time, and dilution buffer can all be altered to optimize staining.
6) Examine protocols for optimal results

All immunostaining protocols require careful optimization, factors to consider include the nature of the blocking buffer, antibody diluents and wash solutions, the length of any incubation steps, and the method of detection. While some primary antibodies produce clear staining after a short incubation at room temperature, others perform better with an overnight incubation at 4oC. Sample preparation is also important, with some antibodies recognizing an epitope in its native state and others binding only when the sample has been denatured. By studying the protocols provided by the manufacturer as well as researching the literature, the desired pattern of staining should be achievable.
7) Handle the antibody correctly
Refer to the product datasheet to determine how to best reconstitute, store, and aliquot the antibody. The datasheet will also detail any special considerations, for example indicating that fluorophore-conjugated antibodies should always be stored and incubated in the dark.
8) Always include relevant experimental control
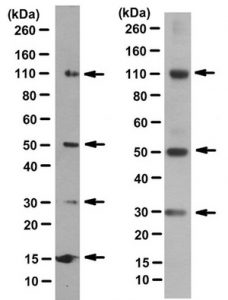 Sensible interpretation of data is reliant on the inclusion of positive and negative controls.Positive controls may include a recombinant protein, or a lysate prepared from a cell line known to produce detectable levels of the target. Negative controls might include knockout samples, or lysates prepared from siRNA-treated cells. Controls to ensure that antibodies are behaving as expected are also vital. Omission of the primary antibody allows background staining attributable to the secondary antibody to be assessed, while isotype controls (antibodies that do not recognize the target, but that share the same isotype as the primary antibody) can be used to identify background staining that is due to the primary antibody.
Sensible interpretation of data is reliant on the inclusion of positive and negative controls.Positive controls may include a recombinant protein, or a lysate prepared from a cell line known to produce detectable levels of the target. Negative controls might include knockout samples, or lysates prepared from siRNA-treated cells. Controls to ensure that antibodies are behaving as expected are also vital. Omission of the primary antibody allows background staining attributable to the secondary antibody to be assessed, while isotype controls (antibodies that do not recognize the target, but that share the same isotype as the primary antibody) can be used to identify background staining that is due to the primary antibody.




Leave a reply