Protein pull-down is a well established technology in laboratories. This procedure can be difficult, particularly when the protein of interest is expressed at low levels. In this blog post we discuss 5 tips to help you improve your IP results.
Problems related to protein concentration
 In general, the concentration of the protein of interest is unknown when immunoprecipitation is performed; however, the performance of a IP depends on the concentration of that protein: the higher the protein concentration, the higher the IP yield. We estimate that the intracellular protein concentration is approximately 1 nM for low expression level, approximately 50 nM for endogenous protein expression levels, and approximately 1000 nM for highly expressed proteins, respectively; all this depends on the type of cell.
In general, the concentration of the protein of interest is unknown when immunoprecipitation is performed; however, the performance of a IP depends on the concentration of that protein: the higher the protein concentration, the higher the IP yield. We estimate that the intracellular protein concentration is approximately 1 nM for low expression level, approximately 50 nM for endogenous protein expression levels, and approximately 1000 nM for highly expressed proteins, respectively; all this depends on the type of cell.
Binding-related problems
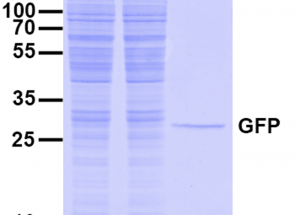
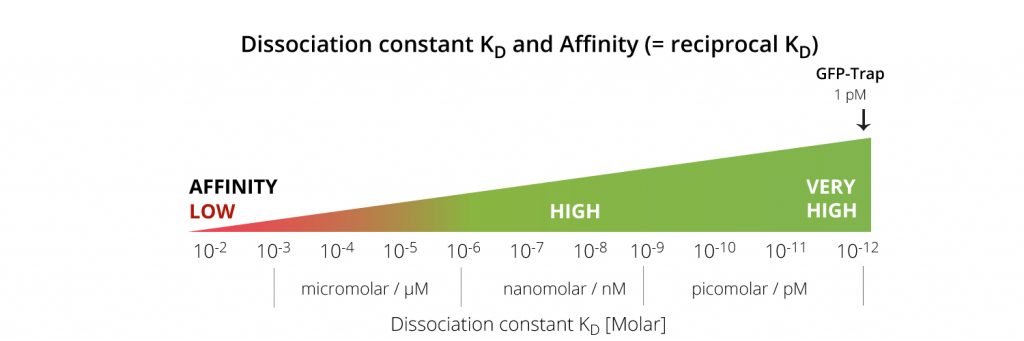
An antibody that is specific for recognizing a protein may not work well when it immunoprecipitates that protein at low concentrations. Make sure your antibody has a low KD (dissociation constant) because low affinity antibodies bind only to a very small protein fraction. The higher the binding affinity of the IP resin, the higher the protein yield. For example: At a protein concentration of 50 nM, a low affinity medium IP resin with a KD of 150 nM binds only 25% of the protein, while a high affinity resin with a KD of 1 nM binds binds almost all, that is, 98% of the protein. Chromotek’s GFP-Trap has a remarkably low KD of just 1 pM (very high affinity) and therefore enables efficient pull-downs of even low protein concentrations.
 Time-related issues
Time-related issues
The optimal wash time must be determined experimentally. It is necessary to consider the effective binding of the protein of interest (for Co-IP, the formation of the protein complex), the degradation of the protein over time and the elimination of background noise by multiple washing steps. Use high affinity IP resins when the protocol requires multiple and prolonged washes. However, the use of IP resin with low dissociation rates helps prevent leaching of the bound protein. Using an IP resin with high binding rates shortens incubation times. The shorter the wash time, the better the pull-down performance.
Specificity-related issues
The better the specificity of the matrix, the lower the background noise. Use only highly specific, well characterized and validated IP resins for reliable and reproducible results.
Volume issues
The lower the volume, the more effective your IP will be. Using small volumes maintains a high protein concentration and therefore increases binding performance. The concentration is a function of the volume. Try to use as small a volume as possible. That keeps your protein concentration high and therefore increases binding efficiency.
In summary, we recommend considering protein concentration, binding affinity, processing time, IP resin specificity, and buffer volume when planning your next immunoprecipitation for the best immunoprecipitation results.
GFP-Trap for Immunoprecipitation
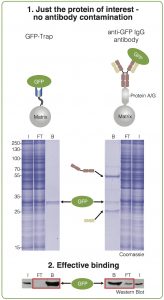
Profits:
• No presence of heavy and light chains.
• Very high binding affinity (KD = 1 pM).
• Short incubation times of 5 to 30 minutes.
• Reproducible results.
• Extraordinary stability in harsh washing conditions.
GFP-Trap is the Gold Standard
Over 1,600 articles have already been published using ChromoTek GFP-Trap. GFP-Trap is the gold standard for IP of GFP fusion proteins. For references, click here.




Leave a reply