We are not aware of it, but we breathe between 7 and 8 liters of air per minute. That air, apart from the oxygen with which we live, also contains dust particles, pollutants and possible pathogens. It is the job of the epithelial cells in our airways to build a barrier and make sure that those agents are quickly removed so as to avoid damage to our lungs. However, pulmonary diseases such as asthma or chronic obstructive pulmonary diseases (COPD) impair this “cleaning” ability of the airway epithelium, and the consequences can be very severe. ALI
In order to study what happens in our airways, researchers need to replicate the in vivo environment as closely as possible. With new commitments to decrease the use of animals in research, a search for models that are physiologically relevant is growing. Air-liquid interface cell culture (ALI) mimic the characteristics of airway epithelial cells and are an optimal alternative to animal studies.
ALI systems provide relevant information on the physiological and pathological processes of the respiratory tract, which can be applied to a wide range of research, such as the fight against infectious agents, the effects of pollution or the effect of drug treatment. Even so, like any in vitro system, it has different challenges to overcome in order to be validated and standardized with reproducible results.
Air-liquid interface cell culture (ALI): replicating the anatomy of human lungs in vitro
The 80m2 of lung surface are composed of different cell types, goblet cells, basal cells and ciliated epithelial cells, among others (Miller and Spence, 2017). This is due to the different tasks that the epithelium of the airways must carry out. Deal with up to 25 million inhaled particles. Goblet cells and seromucous glands produce mucus, and this viscoelastic gel forms a protective layer that traps microorganisms.
The mucociliary mechanism transports any foreign particles to the mouth to clear them away. (Rogers, 2007). Epithelial cells produce various cytokines that participate in the mechanisms of the innate and adaptive immune response. (Mertens et al., 2017).

Due to the large number of tasks performed by epithelial cells, establishing an in vitro system that works in a similar way is difficult. 2D cultures have several disadvantages, such as loss of tissue architecture, lack of cell differentiation, and damage in barrier integrity. All of these features are very important when investigating respiratory diseases. In 2D in vitro cultures, where cells show rapid dedifferentiation, high senescence, and low proliferation (Ramirez et al., 2004).
For all these reasons, 3D cultures of human airway epithelial cells have recently been gaining interest. ALI culture systems manage to overcome many of the challenges of 2D cultures and have many advantages when compared to submerged culture systems. ALI cultures exhibit a pseudostratified morphology, undergo mucociliary differentiation, secrete mucin and form tight junctions. Also, the extracellular environment is similar to the in vivo conditions. (Lacroix et al., 2018).
ALI cultures: powerful models of the human airways
Cell cultures performed at the air-liquid interface facilitate the establishment of stable and functional 3D models of the respiratory tract In these models, the basal side of the cells is in contact with the culture medium, and the apical side is in contact with the air. Therefore, the created in vitro scenario is close to what occurs in vivo.
To establish ALI cultures, primary epithelial cells are first seeded on plastic culture vessels, where they can expand until they reach 70-80% confluence. After this first 2D expansion phase, cells are transferred to compartmentalized culture systems on porous membranes. Here, cells are submerged in culture medium and start proliferating in 3D until reaching confluence. Finally, they are ‘air-lifted,’ i.e. exposed to the surrounding air, and nutritive supply is provided only at the basolateral cell pole. This system allows morphological and functional cell differentiation and the formation of a pseudostratified epithelium with full basoapical polarity.
The reconstituted epithelium shows many features observed in vivo, such as the production of cilia and mucus (Chen and Schoen, 2019).
Standardization: a key requirement for creating relevant culture systems
Standardization and validation of methods based on human material become even more important when research results have clinical relevance. Using defined culture media and pre-screened cells is a concrete step forward towards the establishment of such standardized cultures. The use of serum and bovine pituitary extract (BPE)-free culture media allows the generation of predictive and reproducible 3D airway model systems and offers several advantages, such as:
- Defined and controlled culture conditions
- Reduced variability in qualitative and quantitative culture medium composition
- Reduced risks of microbial contamination
- Higher resemblance of in vivo physiological conditions
- Reduction or even avoidance of the suffering of fetuses and animals
Heterogeneity in results is common when you are performing experiments with primary cells. For example, a lack of barrier formation, this may occur due to donor variability, since bronchial epithelial cells from donors who have been under chronic medication have a very permeable barrier function.
To solve this problem, researchers can use preselected primary cells that have been tested specifically for ALI cultures. These ensure high and stable TEER(Transepithelial electrical resistance) values and therefore an optimal barrier function. The preselected cells can also be used as standard positive controls to compare experimental results.
Since the epithelial cells of the airways are involved in immunological processes of inflammation and tolerance, the expression of human leukocyte antigen (HLA) in these cells is relevant information for research. Therefore, the use of HLA-like cells could be very useful when researchers wish to establish standardizedALI cultures for respiratory research.
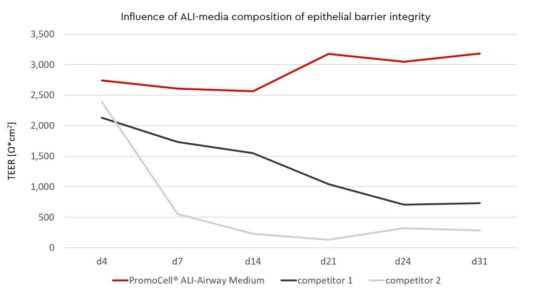
Compositions of Air-Liquid Interface (ALI) media influence epithelial barrier integrity
Selecting an appropriate medium is a crucial step in developing an ALI culture with long-lasting barrier function.
Epithelial cell barrier formation can be quantitatively measured using transepithelial electrical resistance (TEER) with a voltohmmeter. To establish ALI cultures you can use the Air-Liquid Interface (ALI-Airway) Medium, of Promocell, the cells (Human Bronchial Epithelial Cells -HBEpCneed to seed on collagen type I coated Transwell® inserts and be allowed to grow to 100% confluence in epithelial cell growth medium of the airways in submerged culture.
Seeding of cells in early passage is best, with passage 3 giving optimal results. After reaching confluence, the medium should be switched to the ALI-Airway Medium and cells in the inserts should be exposed to air (airlift).
To monitor barrier formation in real time the voltohmmeter with STX2 Chopstick® electrode set can be used without damaging the cells.
In the first week of ALI-culture you can achieve TEER values >100Ω*cm², values that have been shown to be physiologically relevant in rabbit airway epithelium (Bath, 1993) and thanks to the ALI-Airway medium, the barrier maintains a stable level.
In the second week of ALI contact, TEER values are achieved > 500 Ω*cm2 for HBEpC.
IEven in week 3 and 4 after starting the ALI-culture barrier integrity will not decline under a threshold of > 500 Ω*cm2. Optimal barrier integrity with a high degree of viable cells (> 70 % viability) will be available for at least four weeks. The choice of the right ALI-medium is therefore essential for successful ALI-cultures.
Using air-liquid systems with epithelial cells to understand lung diseases
Pulmonary diseases such as chronic obstructive pulmonary disease (COPD), asthma, pneumonia and lung cancer have a huge global impact. ALI systems use primary human cells as a model for such diseases and allow a better understanding of the underlying pathological changes. oreover, cultured cells can be exposed to pathogens, aerosolized medications and toxic substances, and air pollution can be used to model phases of acute exacerbation or observe the effects of therapeutic drugs (Mertens et al., 2017).
In a recent study, Mulay et al. isolated human epithelial cells from trachea and upper bronchi and differentiated them at ALI for 16–20 days until a pseudostratified mucociliary epithelium was formed. The team then infected these ALI cultures with SARS-CoV-2 and observed virus replication and gene expression in infected cells. The proximal airway ALI cultures, together with 3D alveolar organoids, were also used to study the effect of a selected panel of drugs such as IFNB1, Remdesivir and Hydroxychloroquine on viral infection and replication.

The advantage of ALI systems is that they provide a method to challenge your cultured epithelium with any agent of interest. Either air pollutants, toxic substances, viruses, bacteria or drugs can be used to observe the reactions of the cells to these agents. The system is a valid alternative to in vivo experiments or classic in vitro methods.
If you want to know more about ALI systems or want to try Promocell media in your cultures, do not hesitate to write to us using the following form:









Leave a reply